General Acid Base Titration Equation . calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. there are two basic types of acid base titrations, indicator and potentiometric. By the end of this section, you will be able to: hcl + naoh → nacl + h 2 o.
from general.chemistrysteps.com
hcl + naoh → nacl + h 2 o. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric.
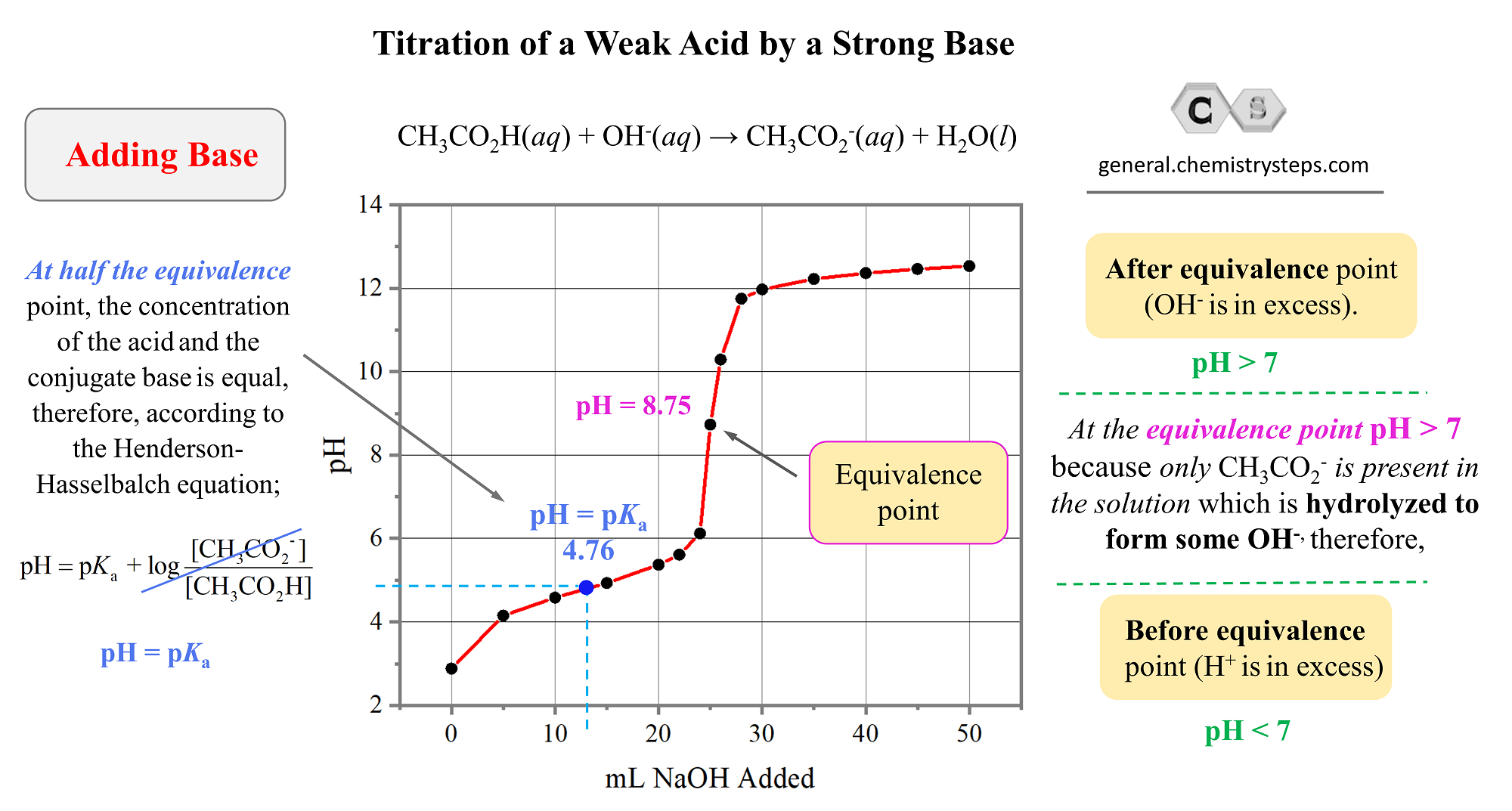
Titration of a Weak Acid by a Strong Base Chemistry Steps
General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. there are two basic types of acid base titrations, indicator and potentiometric. By the end of this section, you will be able to: hcl + naoh → nacl + h 2 o.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. General Acid Base Titration Equation.
From studykaki.com
AcidBase Titration Study notes, tips, worksheets, exam papers General Acid Base Titration Equation By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. General Acid Base Titration Equation.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle General Acid Base Titration Equation By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. General Acid Base Titration Equation.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. General Acid Base Titration Equation.
From mungfali.com
Acid Base Titration Calculation General Acid Base Titration Equation By the end of this section, you will be able to: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. there are two basic types of acid base titrations, indicator and potentiometric. General Acid Base Titration Equation.
From www.vrogue.co
Sketch A Properly Labeled Titration Curve For The Che vrogue.co General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: General Acid Base Titration Equation.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: General Acid Base Titration Equation.
From www.studypool.com
SOLUTION Acid base titration Studypool General Acid Base Titration Equation By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. hcl + naoh → nacl + h 2 o. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. General Acid Base Titration Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube General Acid Base Titration Equation calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. there are two basic types of acid base titrations, indicator and potentiometric. hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: General Acid Base Titration Equation.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. By the end of this section, you will be able to: hcl + naoh → nacl + h 2 o. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. General Acid Base Titration Equation.
From fity.club
Titration Formula General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: General Acid Base Titration Equation.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators General Acid Base Titration Equation calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: hcl + naoh → nacl + h 2 o. there are two basic types of acid base titrations, indicator and potentiometric. General Acid Base Titration Equation.
From mungfali.com
Acid Base Titration Calculation General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. General Acid Base Titration Equation.
From mungfali.com
Acid Base Titration Calculation General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: General Acid Base Titration Equation.
From mungfali.com
Acid Base Titration Formula General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. By the end of this section, you will be able to: General Acid Base Titration Equation.
From www.youtube.com
17.3a Strong Acid Strong Base Titrations pH Calculations General General Acid Base Titration Equation there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: General Acid Base Titration Equation.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation General Acid Base Titration Equation By the end of this section, you will be able to: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. there are two basic types of acid base titrations, indicator and potentiometric. hcl + naoh → nacl + h 2 o. General Acid Base Titration Equation.
From mungfali.com
Acid Base Titration Calculation General Acid Base Titration Equation hcl + naoh → nacl + h 2 o. By the end of this section, you will be able to: there are two basic types of acid base titrations, indicator and potentiometric. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. General Acid Base Titration Equation.